Chimeric RNA Driven Neopeptide Vaccine for Prevention of Breast Cancer in Germline BRCA1/2 Carriers
PI: Isabelle Bedrosian, MPI: Anjana Bhardwaj, MPI: Preethi Gunaratne
Institutions: MD Anderson Cancer Center, University of Houston
Project Number: UG3CA290454
The main goal of this research is to develop a new immunoprevention strategy that can protect individuals with germline BRCA1/2 mutations from developing breast cancer. People carrying these inherited mutations face a 60 to 80 percent lifetime risk of disease, and current management options such as prophylactic bilateral mastectomy or intensive MRI surveillance are invasive, burdensome, and often associated with significant physical and psychological costs. This project aims to provide a low-toxicity alternative by using new advances in genomics, bioinformatics, and vaccine science. The central idea is that chimeric mRNAs found in BRCA1/2-related breast tissue generate novel, immunogenic neoantigens that can be targeted by preventive vaccines long before cancer forms.
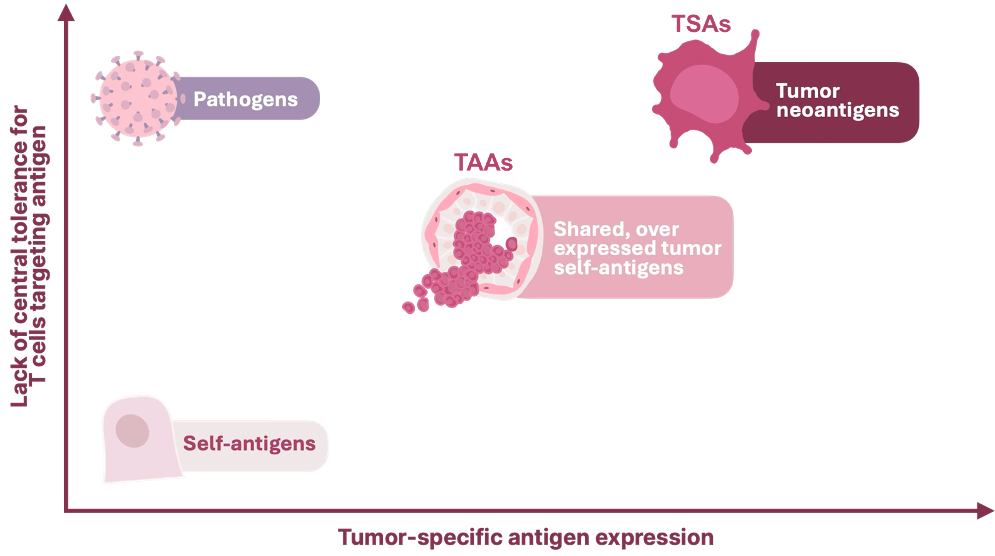
This research provides an important opportunity to define the antigenic landscape of preneoplastic breast tissue and convert this knowledge into a vaccine-based approach for cancer interception. By combining sequencing technologies with computational peptide prediction, the team seeks to identify fusion-derived proteins that arise during the earliest stages of tumor development. These neoantigens, which are present in both high-risk breast tissue and established tumors, may serve as ideal targets for an off-the-shelf vaccine designed for people predisposed to BRCA1/2-driven cancers.
The research will pursue the following aims:
Scientific Focus within CIP-Net
This project aligns closely with the CIP-Net mission to transform discoveries in immune biology into preventive interventions for individuals at elevated cancer risk. By identifying early antigenic events in BRCA1/2-associated tumorigenesis and connecting them to actionable vaccine strategies, the study represents a clear example of precision immunoprevention. It uses advanced genomic and computational tools to intercept cancer at its earliest stages, supporting CIP-Net’s goals in early detection, biological insight, and immune-based cancer prevention.
Public Health Relevance
This work addresses a major unmet need in hereditary breast cancer prevention by seeking effective and noninvasive options for people with germline BRCA1/2 mutations. By integrating advances in sequencing, informatics, and genomic therapies, the project introduces a new approach to primary cancer prevention. If successful, this off-the-shelf immunoprevention vaccine could reduce the incidence of BRCA-associated breast cancer and may be adaptable to other hereditary cancers caused by high-penetrance mutations. The study supports broader efforts to shift cancer control from treatment to prevention through early interception and immune-based approaches.
This research provides an important opportunity to define the antigenic landscape of preneoplastic breast tissue and convert this knowledge into a vaccine-based approach for cancer interception. By combining sequencing technologies with computational peptide prediction, the team seeks to identify fusion-derived proteins that arise during the earliest stages of tumor development. These neoantigens, which are present in both high-risk breast tissue and established tumors, may serve as ideal targets for an off-the-shelf vaccine designed for people predisposed to BRCA1/2-driven cancers.


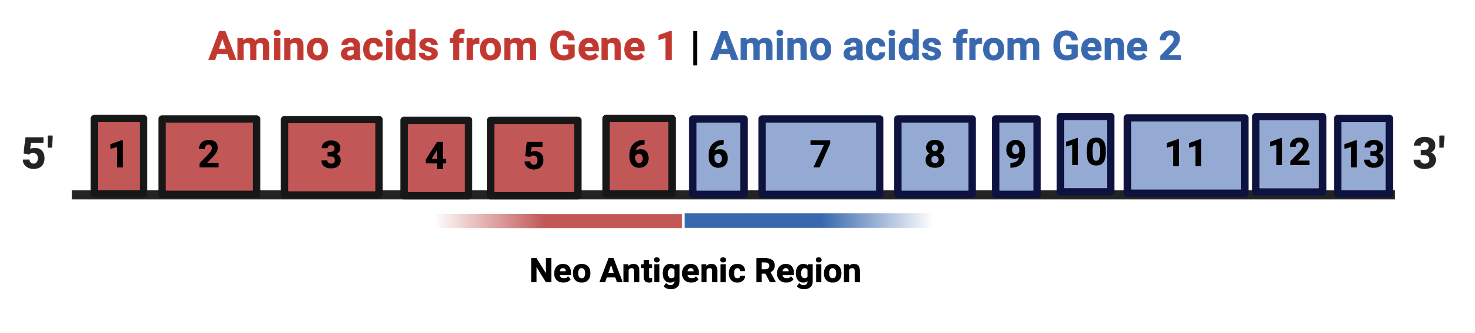
Chimeric RNA/ RNA fusions: source of neoantigens
The research will pursue the following aims:
- Aim 1. Identify and validate MHC Class I immunogenic neopeptides from BRCA 1 /2 patients, BRCA1 mutant mice and a list of shared candidates.
- Aim 2. Determine the efficacy of neoantigens derived from chimeric mRNA as a vaccine for prevention of breast cancer in BRCA 1 mouse model of breast cancer.
- Aim 3. Delineate the mechanisms of immunoprevention by neoantigen vaccine in BRCA1 mouse model of breast cancer.
Scientific Focus within CIP-Net
This project aligns closely with the CIP-Net mission to transform discoveries in immune biology into preventive interventions for individuals at elevated cancer risk. By identifying early antigenic events in BRCA1/2-associated tumorigenesis and connecting them to actionable vaccine strategies, the study represents a clear example of precision immunoprevention. It uses advanced genomic and computational tools to intercept cancer at its earliest stages, supporting CIP-Net’s goals in early detection, biological insight, and immune-based cancer prevention.
Public Health Relevance
This work addresses a major unmet need in hereditary breast cancer prevention by seeking effective and noninvasive options for people with germline BRCA1/2 mutations. By integrating advances in sequencing, informatics, and genomic therapies, the project introduces a new approach to primary cancer prevention. If successful, this off-the-shelf immunoprevention vaccine could reduce the incidence of BRCA-associated breast cancer and may be adaptable to other hereditary cancers caused by high-penetrance mutations. The study supports broader efforts to shift cancer control from treatment to prevention through early interception and immune-based approaches.

Isabelle Bedrosian, MD
University of Texas MD Anderson Cancer Center

Anjana Bhardwaj, PhD
University of Texas MD Anderson Cancer Center

Preethi Gunaratne, PhD
University of Houston

Janvi Sandhu
University of Texas MD Anderson Cancer Center

Shiyanth Thevasagayampillai, PhD Candidate
University of Houston

Aaranyah Kandasamy, PhD Candidate
University of Houston

Dilshan Adhikari, PhD Candidate
University of Houston

Brandon Than, BS
University of Houston

Sanjula Rupasinghe
